Do you have any questions?

Elastron offers its V and G series of compounds which meet the special needs of various medical device applications.
Elastron also offers biocompatibility while exhibiting high performance through eco-friendly solutions.
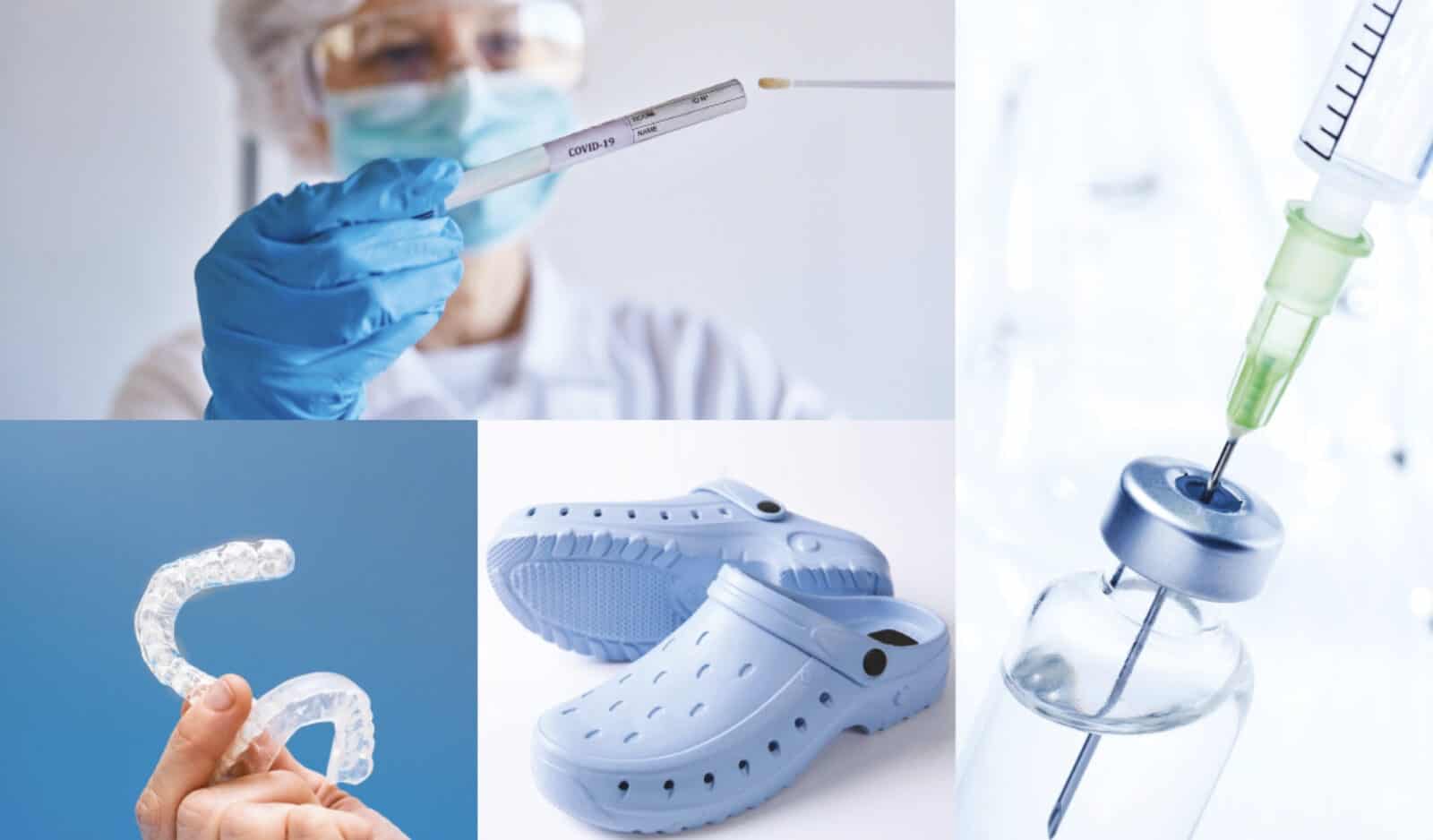
Segment Applications
Medical Footwear
- Hospital clogs
Medical Equipment & Devices
- Dental Guards for Bruxism Disease
- Peristallic Pump Tubes
- Urine Catheter Grips
Medical Tips & Gaskets
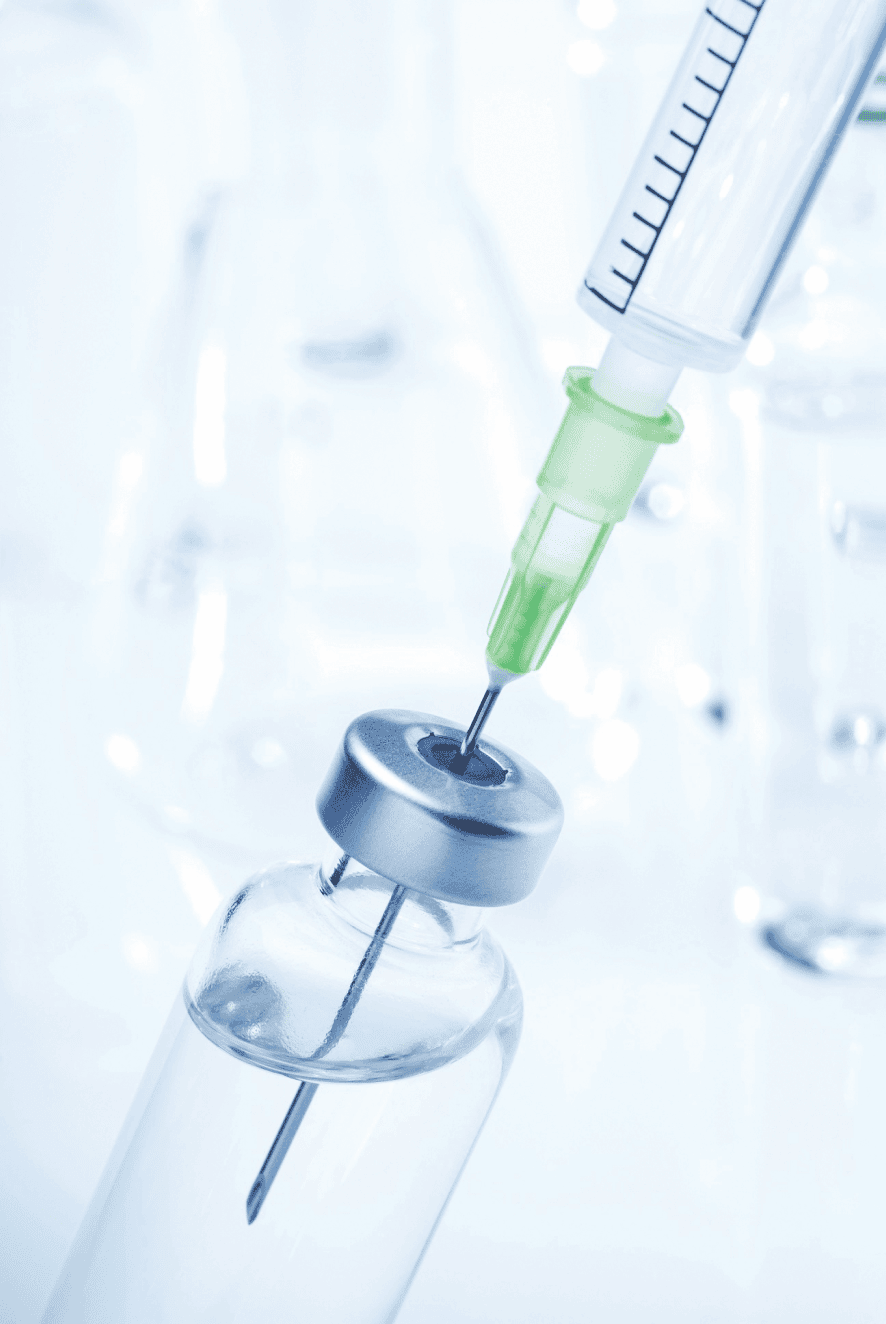
- Infusion Bottle Caps
- Syringe Gaskets
Dispoable Medical Goods
- Brush of Throat Swabs
Documents
Key Features of Elastron TPE Grades for Medical
- Excellent sealing property and good chemical resistance
- Suitable for sterilization with gamma irradiation, ethylene oxide (EtO) and steam
- Suitable for extrusion/co-extrusion, injection/co-injection and overmolding
- Regulation (EU) No.10/2011, ISO 10993-5, FDA 21 CFR raw material, USP <88> and <87> conformity
- Adhesion to polyolefins and EVA
- Free from; animal derivates, heavy metals, bisphenol A, PVC, latex, silicone and nitrosamine